Single-cell RNA sequencing (scRNA-seq) helps us understand the complexity of cells at a single-cell level. The Seurat single-cell RNA-seq analysis pipeline 2024 offers an updated, flexible way to explore and analyze this data. Whether you’re a beginner or an advanced user, this guide will walk you through the main steps, from data loading to advanced visualization, with scenarios to demonstrate the flexibility of Seurat.
Introduction to Seurat and scRNA-Seq Analysis
The Seurat single-cell RNA-seq analysis pipeline 2024 is an essential tool for analyzing gene expression data from individual cells. It’s designed to handle large datasets, perform clustering, identify different cell types, and explore relationships between cells. In this article, we’ll explore the basics, followed by code snippets to help you get started.
Key Seurat Updates in 2024
- Improved memory handling for large datasets
- Enhanced visualization options for more complex data
- Integration with new machine learning techniques
Installing Seurat
To use the Seurat single-cell RNA-seq analysis pipeline 2024, make sure you have the latest version of R installed. You can install Seurat directly from CRAN:
install.packages("Seurat")
Or, if you want the development version:
devtools::install_github("satijalab/seurat", ref = "develop")Scenario 1: Filtering Low-Quality Cells
In this first scenario, we will start by loading a sample scRNA-seq dataset and filtering out low-quality cells that can affect the analysis. Here is how to load your data in the Seurat single-cell RNA-seq analysis pipeline 2024:
Step 1: Load the Data
First, load the dataset into a Seurat object.
# Load Seurat package
library(Seurat)
# Load the dataset (assuming data is in 10X format)
data <- Read10X(data.dir = "path/to/data")
# Create a Seurat object with basic filtering
seurat_obj <- CreateSeuratObject(counts = data, project = "LowQuality_Filtering", min.cells = 3, min.features = 200)
Step 2: Perform Quality Control (QC)
Now, apply quality control to filter out cells with low gene counts or high mitochondrial content.
# Calculate mitochondrial gene percentage
seurat_obj[["percent.mt"]] <- PercentageFeatureSet(seurat_obj, pattern = "^MT-")
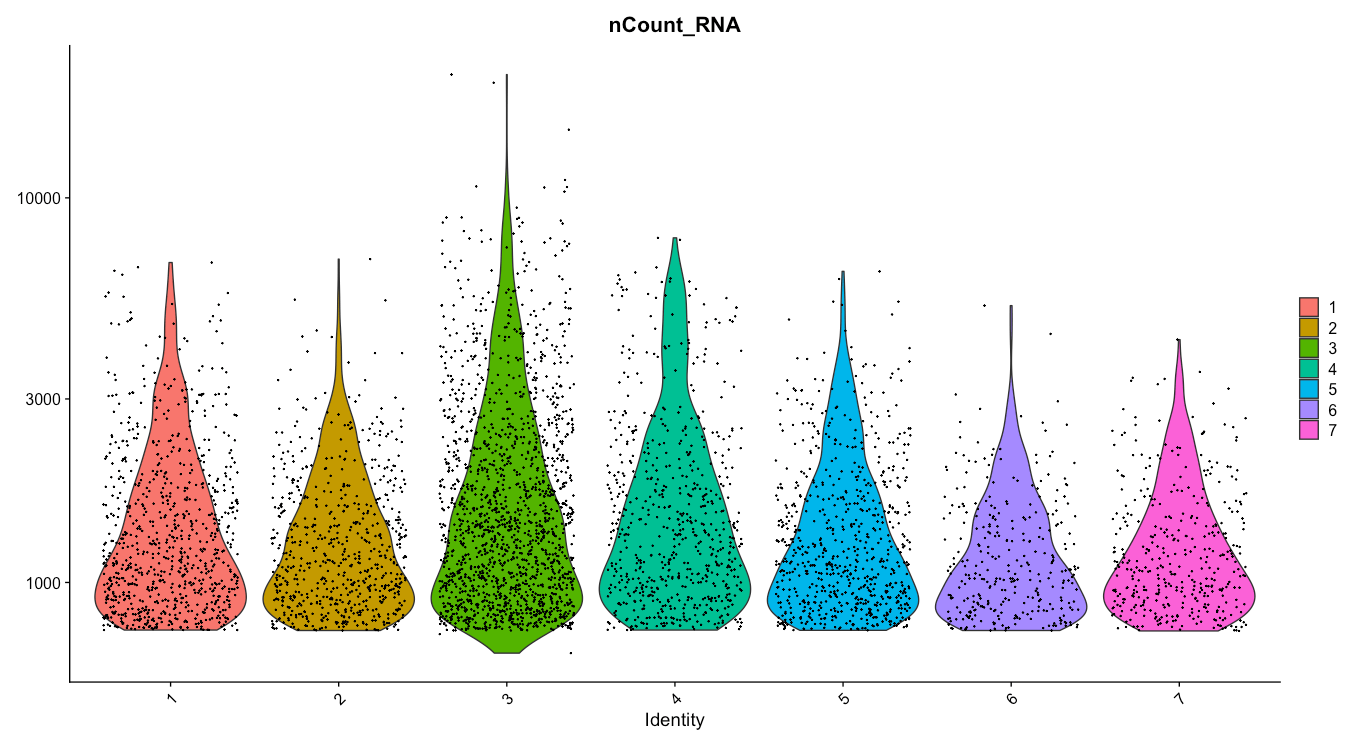
# Visualize QC metrics
VlnPlot(seurat_obj, features = c("nFeature_RNA", "nCount_RNA", "percent.mt"), ncol = 3)
# Filter out low-quality cells
seurat_obj <- subset(seurat_obj, subset = nFeature_RNA > 200 & nFeature_RNA < 2500 & percent.mt < 5)
Step 3: Normalize the Data
Once we have high-quality cells, normalize the data to correct for differences in sequencing depth.
# Normalize the data
seurat_obj <- NormalizeData(seurat_obj)
Step 4: Identify Highly Variable Features
Highly variable genes are essential for downstream clustering and analysis.
# Identify highly variable features
seurat_obj <- FindVariableFeatures(seurat_obj, selection.method = "vst", nfeatures = 2000)
Step 5: Scale the Data
Next, scale the data to remove unwanted sources of variation.
# Scale the data
seurat_obj <- ScaleData(seurat_obj)
Step 6: Perform Principal Component Analysis (PCA)
PCA reduces the dimensionality of the data, making it easier to cluster cells.
# Run PCA
seurat_obj <- RunPCA(seurat_obj, features = VariableFeatures(object = seurat_obj))
# Visualize PCA results
ElbowPlot(seurat_obj)
Step 7: Cluster the Cells
We use clustering to group similar cells together.
# Find clusters using a resolution parameter (adjust based on data size)
seurat_obj <- FindNeighbors(seurat_obj, dims = 1:10)
seurat_obj <- FindClusters(seurat_obj, resolution = 0.5)
Step 8: Visualize the Clusters
Finally, we use UMAP or t-SNE to visualize the clusters.
# Run UMAP for visualization
seurat_obj <- RunUMAP(seurat_obj, dims = 1:10)
# Plot the UMAP clusters
DimPlot(seurat_obj, reduction = "umap")
Scenario 2: Comparing Healthy vs. Diseased Samples
In this second scenario, we will use the Seurat single-cell RNA-seq analysis pipeline 2024 to compare healthy and diseased samples.
Step 1: Load and Merge the Datasets
We load two datasets (healthy and diseased) and merge them into a single object.
# Load healthy and diseased data
healthy_data <- Read10X(data.dir = "path/to/healthy")
diseased_data <- Read10X(data.dir = "path/to/diseased")
# Create Seurat objects
healthy_obj <- CreateSeuratObject(counts = healthy_data, project = "Healthy")
diseased_obj <- CreateSeuratObject(counts = diseased_data, project = "Diseased")
# Merge datasets into one object
merged_obj <- merge(healthy_obj, y = diseased_obj, add.cell.ids = c("Healthy", "Diseased"), project = "Merged_Comparison")
Step 2: Normalize the Data
We normalize the merged dataset.
# Normalize the merged dataset
merged_obj <- NormalizeData(merged_obj)
Step 3: Identify Variable Features
Highly variable features are critical for meaningful comparisons between conditions.
# Find variable features
merged_obj <- FindVariableFeatures(merged_obj)
Step 4: Scale the Data
We scale the data to remove unwanted sources of variation.
# Scale the data
merged_obj <- ScaleData(merged_obj)
Step 5: Perform Dimensionality Reduction (PCA)
We reduce the dimensions of the dataset using PCA.
# Run PCA
merged_obj <- RunPCA(merged_obj, features = VariableFeatures(object = merged_obj))
# Visualize PCA results
ElbowPlot(merged_obj)
Step 6: Cluster the Cells
We cluster cells based on their expression profiles.
# Find clusters
merged_obj <- FindNeighbors(merged_obj, dims = 1:10)
merged_obj <- FindClusters(merged_obj, resolution = 0.5)
Step 7: Identify Differentially Expressed Genes
We compare healthy vs. diseased cells to find differentially expressed genes.
# Identify differentially expressed genes
diff_genes <- FindMarkers(merged_obj, ident.1 = "Healthy", ident.2 = "Diseased")
head(diff_genes)
Step 8: Visualize the Clusters
Finally, visualize the differences between clusters using UMAP.
# Run UMAP
merged_obj <- RunUMAP(merged_obj, dims = 1:10)
# Plot UMAP
DimPlot(merged_obj, reduction = "umap", split.by = "orig.ident")
Scenario 3: Integrating Multiple Datasets
In the third scenario, we will integrate multiple datasets using the Seurat single-cell RNA-seq analysis pipeline 2024. This is useful when you have data from different batches or experiments that need to be analyzed together.
Step 1: Load Multiple Datasets
We load multiple datasets that we want to integrate.
# Load two datasets from different batches
data1 <- Read10X(data.dir = "path/to/data1")
data2 <- Read10X(data.dir = "path/to/data2")
# Create Seurat objects for each dataset
obj1 <- CreateSeuratObject(counts = data1)
obj2 <- CreateSeuratObject(counts = data2)
Step 2: Normalize and Identify Variable Features
We normalize and identify variable features for each dataset separately.
# Normalize datasets and identify variable features
obj1 <- NormalizeData(obj1)
obj2 <- NormalizeData(obj2)
obj1 <- FindVariableFeatures(obj1)
obj2 <- FindVariableFeatures(obj2)
Step 3: Find Integration Anchors
We identify common features (anchors) between the datasets to align them.
# Find integration anchors
anchors <- FindIntegrationAnchors(object.list = list(obj1, obj2))
Step 4: Integrate the Data
We integrate the datasets to remove batch effects.
# Integrate data
integrated_obj <- IntegrateData(anchorset = anchors)
Step 5: Scale the Integrated Data
We scale the integrated data to ensure consistency.
# Scale the integrated data
integrated_obj <- ScaleData(integrated_obj)
Step 6: Perform Dimensionality Reduction
We reduce the dimensions of the integrated data.
# Run PCA
integrated_obj <- RunPCA(integrated_obj)
# Visualize the Elbow plot to choose significant PCs
ElbowPlot(integrated_obj)
Step 7: Cluster the Cells
We cluster the integrated data to identify groups of cells.
# Find clusters
integrated_obj <- FindNeighbors(integrated_obj, dims = 1:20)
integrated_obj <- FindClusters(integrated_obj, resolution = 0.5)
Step 8: Visualize the Clusters
Finally, we visualize the clusters using UMAP or t-SNE.
# Run UMAP and visualize clusters
integrated_obj <- RunUMAP(integrated_obj, dims = 1:20)
# Plot UMAP
DimPlot(integrated_obj, reduction = "umap")Conclusion
The Seurat single-cell RNA-seq analysis pipeline 2024 offers a flexible and powerful approach to analyzing scRNA-seq data. Whether you are filtering low-quality cells, comparing different conditions, or integrating multiple datasets, Seurat provides tools to make complex analyses easier. Each scenario in this guide demonstrates how the pipeline can be adapted for different datasets, helping you uncover valuable insights in your research.
It’s clear that you are passionate about making a positive impact and your blog is a testament to that Thank you for all that you do
Thank you for your feedback